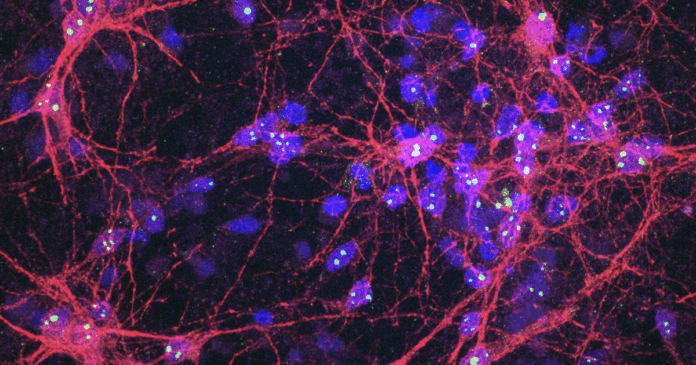
For the first time, a lab-grown brain-computer system has demonstrated that human neurons living and evolving in an artificial system respond to medication by learning, in real time, in a game-like environment. Subjected to anti-seizure drugs, Cortical Labs’ disease-modeled neurons didn’t just show altered brain activity, but spontaneous information-processing behavior. It’s a huge step forward for the company’s synthetic biological intelligence (SBI) technology and how we are able to research neurological conditions and develop new, effective treatments.
“This breakthrough is a major step forward in not only how we study and understand diseases and drugs that are designed to treat related neural processes impacted by these diseases,” said Brett Kagan, Chief Scientific Officer at Cortical Labs. “For the first time, alongside some of the world’s most eminent researchers in their field, we’ve been able to show that impaired information processes of a disease in a dish can be restored using a drug designed specifically to treat it.”
Cortical Labs
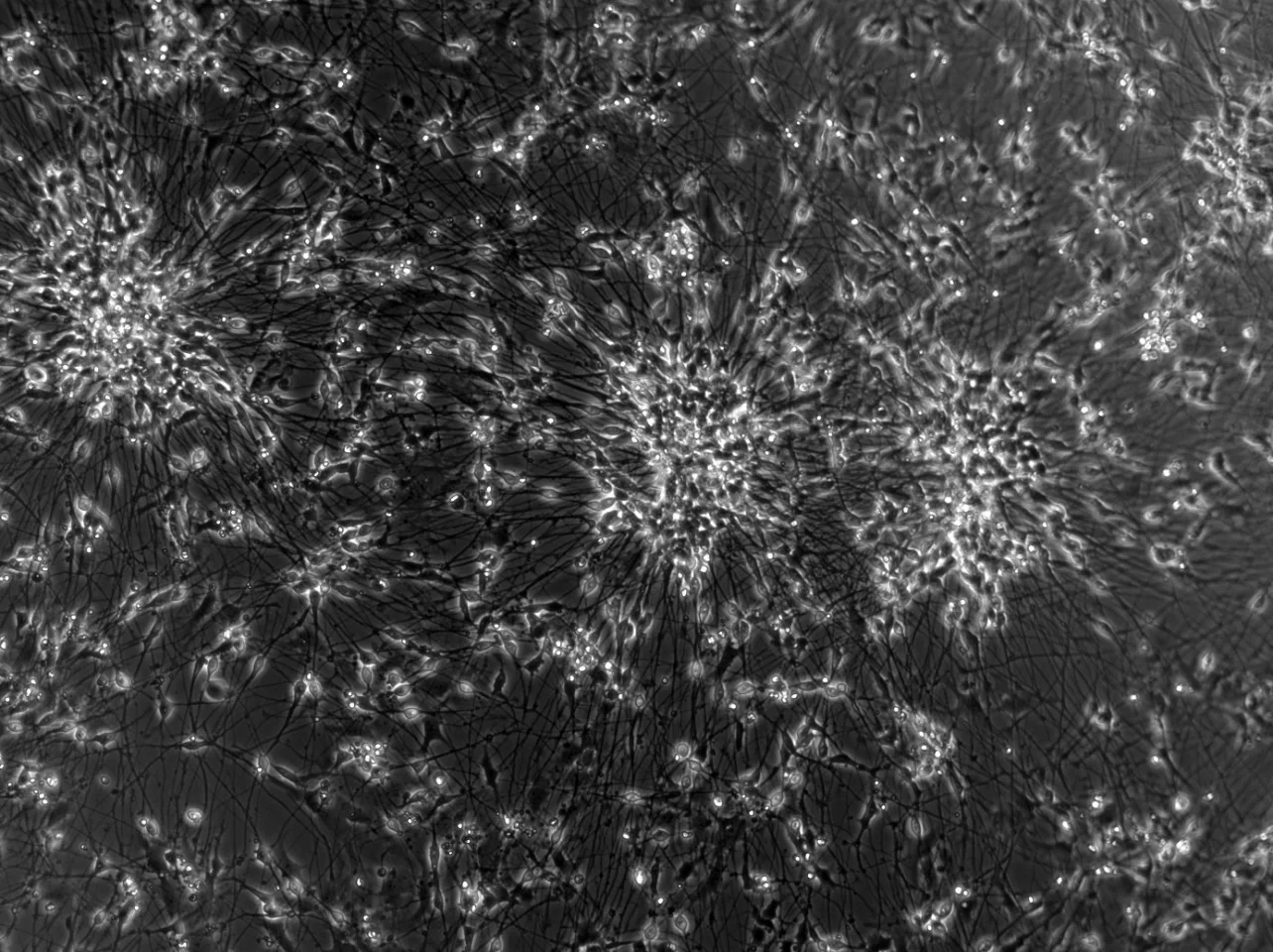
Led by Australia-based Cortical Labs, which we have covered extensively – from the earliest DishBrain advances – the study is the first to demonstrate the real-world potential of the commercial SBI CL1 platform. As extensively covered in our visit to the company’s Melbourne laboratory, the CL1 biological computer features lab-grown neurons from human stem cells on a silicon base in a life-supporting specially designed box. When together, each CL1 forms a rack that resembles a computer server. The neurons form networks and, in response to stimuli, process this information and respond in real time, rerouting or forming new connections in a way that optimizes efficiency and cell health.
These neurons are hooked up to a computer playing a simplified version of Pong, which can test neuronal information processing and changes in real time. As earlier research demonstrated, the SBI “learn” in response to game-play feedback, processing information and adjusting connections to improve performance.

Cortical Labs
Using the CL1 platform, which houses the DishBrain system, the team induced hyperactive glutamatergic dysregulation in human-induced pluripotent stem cells (hiSCs), where a glutamate neurotransmitter imbalance overstimulated the cells and resulted in epilepsy-like faulty neurons.
Following 21 days of glutamatergic neural differentiation, three anti-seizure medications – phenytoin, perampanel and carbamazepine – were tested on the SBI epilepsy model. Seeing how each drug altered neural behavior in real time, the researchers discovered that carbamazepine 200 µM had a significant impact on how the neurons responded, self-regulated and adjusted.
At this high dose, carbamazepine significantly improved the neural system’s ability to play the Pong game. While all three drugs reduced the neurons’ over-firing – epileptic-like bursts of activity – only carbamazepine made the network better at goal-directed behavior. And some neural networks became more stable and organized through treatment.
The research, which also involved University of Cambridge scientists and the UK-based biotech startup bit.bio, is the first to show how disease-modeled SBI changes in response to drug intervention.
“While this is an incredibly significant milestone, and the realization of years of focus at Cortical Labs, it’s only the start,” Kagan said. “The ability to observe how living neurons react to real-time stimulation and drug treatment opens up entirely new ways to develop, test, and personalize therapies – all without relying on animal models. Based on our early findings, we’ll continue to refine the modeling with the aim of developing more effective, patient-specific therapies in the future.”
The systems also allow for the simultaneous testing of experimental drugs and treatments, since the hiSCs in each CL1 box can be grown to have similar cell diversity, or be manipulated to represent different neurological disease models for drug testing. It not only does away with animal testing, but essentially allows for testing of experimental treatments on a human neural network – without the human.
Cortical Labs
While the SBI system is obviously a much simpler model than the human brain, and the drug intervention focused just on glutamatergic neurons, it’s nonetheless a big milestone for Cortical Labs, which has invested years of work to get to this point. As Kagan noted, it’s a great achievement but it’s just another stepping stone in demonstrating the potential of this unique SBI.
“One of the most pressing challenges in neuroscience is improving the success rate of effective new treatments reaching patients,” said Brad Watmuff, Head of Biology at Cortical Labs. “Our work highlights a key obstacle to this goal – that the neural functional endpoints we typically rely on to define treatment efficacy may not be optimal. Importantly, we show these endpoints can be influenced and even improved with drug intervention, opening the door to more meaningful measures of therapeutic success.”
The research was published in the journal Communications Biology.
Source: Cortical Labs via EurekaAlert!