Scientists have developed a powerful new dual-imaging tool that maps the retina’s structure and oxygen use in unprecedented detail. This breakthrough could one day help doctors spot sight-stealing diseases long before symptoms appear.
Our retinas convert light into electrical signals that are transmitted to the brain, where they’re processed into images. It’s a process that requires a great deal of oxygen. If the oxygen supply is disrupted, for example, due to restricted blood flow, it can lead to serious, vision-affecting conditions such as glaucoma, age-related macular degeneration (AMD), and diabetic retinopathy.
In a new study, researchers from Johns Hopkins University and the University of Pennsylvania developed and tested a novel retinal imaging system that combines two cutting-edge techniques to map the retina’s structure and oxygen levels to better study oxygen metabolism.
The researchers’ dual-channel system used visible light optical coherence tomography (VIS-OCT) to capture ultra-detailed structural images of the eye and phosphorescence lifetime ophthalmoscopy (PLIM-SLO) to directly measure oxygen partial pressure (pO2) in the organ’s tiny blood vessels, or microvasculature. In simple terms, pO2 is the amount of oxygen dissolved in the blood at a given location. It is a key indicator of how much oxygen is available to the tissues.
Stephanie Nolen et al. (2025)
These methods were used to image the eyes of live mice. VIS-OCT uses visible light to create high-resolution 3D images of retinal layers and can also capture blood flow dynamics. PLIM-SLO involves injecting a safe, oxygen-sensitive dye called Oxyphor 2P, which emits light that changes depending on oxygen levels. By measuring how quickly this light fades (that is, its phosphorescence lifetime), the researchers could calculate pO2 at the capillary level. Both systems shared the same optical path, allowing them to capture structural and oxygenation data at the same time and in perfect alignment. The researchers also tested how pO2 readings changed as they varied the mice’s inhaled oxygen to validate the novel technique’s accuracy.
PLIM-SLO accurately measured oxygen levels in arterioles, venules, and capillaries. As the researchers had expected, PLIM-SLO revealed that arterioles (very small branches of arteries) had the highest oxygen, venules (the smallest veins that return deoxygenated blood) had the lowest, with capillaries in between. Adjusting the system’s focus allowed the researchers to image oxygen at different retinal depths, revealing the structure and oxygen profile of multiple vascular layers – something that previous methods couldn’t achieve. Changes in inhaled oxygen led to predictable changes in retinal oxygen levels, confirming that measurements reflected real physiological changes. Importantly, the system linked oxygen measurements with structural and flow data, laying the groundwork for future studies of retinal oxygen metabolism and disease processes.
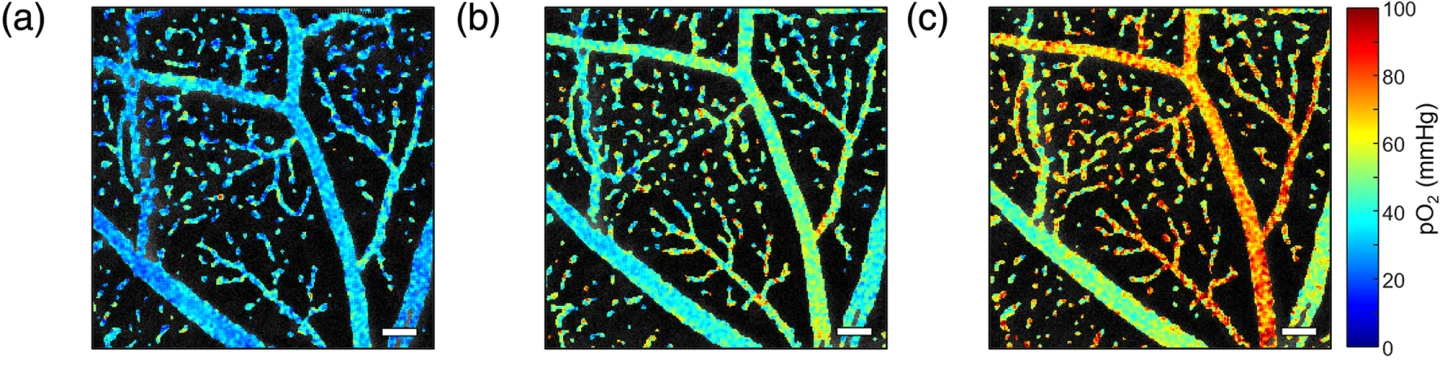
Stephanie Nolen et al. (2025)
Because the system was only tested in mice, its performance in humans hasn’t yet been evaluated. Additional limitations of the study include that the approach requires careful calibration to correct for light interference between the two systems, which is a technical challenge. Also, some physiological factors, such as pH and carbon dioxide levels, had to be estimated rather than directly measured, potentially introducing small errors.
Putting aside these limitations, this multimodal system could significantly advance eye disease research and diagnostics by providing a more complete picture of retinal health. It could help scientists understand how oxygen supply changes in diseases like diabetic retinopathy, glaucoma, and macular degeneration. Clinicians may one day use similar technology to detect early disease-related changes before vision is affected.
The study was financially supported by grants from the National Eye Institute, the National Institute of Biomedical Imaging and Bioengineering, and the National Science Foundation Graduate Research Fellowship Program. It was published in the journal Neurophotonics.
Source: SPIE


